News
29.01.2026
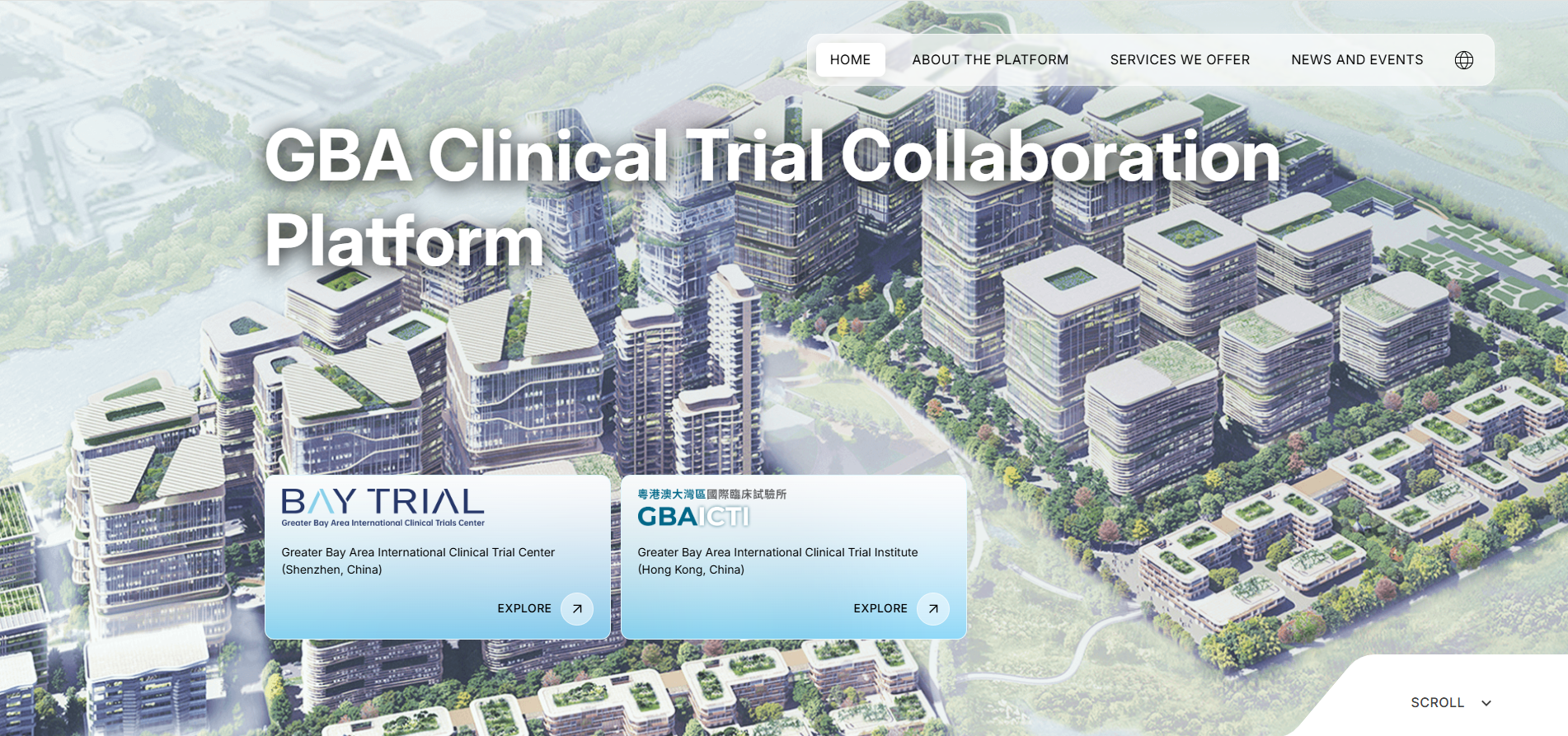
Driving Real‑World Research Forward to Empower Healthcare Innovation Across the GBA
2026 GBA Real-World Research and Application Forum Ends on a High Note
 View More
View More
15.01.2026
Collaboration in Action: The First HK Clinical Trials Roundtable
GBAICTI connects stakeholders to strengthen Hong Kong’s clinical research ecosystem
 View More
View More
22.12.2025
Establishment of Real-World Study and Application Centre and simultaneous launch of GBA Clinical Trial Collaboration Platform
The Greater Bay Area International Clinical Trial Institute (GBAICTI) is pleased to announce the establishment of the Real-World Study and Application Centre (RWSAC), and the launch of the GBA Clinical Trial Collaboration Platform.
 View More
View More
08.12.2025
Career Talk: Discover Your Future in Healthcare & Pharmaceuticals
Join us for an inspiring Career Talk featuring two distinguished HKU alumni, Sara Chan (Clinical Operations Lead, Roche) and Jessie Yip (Project Director, Fortrea), as they share their professional journeys and insights into the dynamic healthcare and pharmaceutical industries.
 View More
View More
21.11.2025
Workshop on Strategic Application of Real-World Data
The Greater Bay Area International Clinical Trial Institute (GBAICTI) proudly convened its exclusive workshop, "Strategic Application of Real-World Data," on November 21, 2025. The event brought together key partners from the pharmaceutical industry for an in-depth exploration of the transformative role of Real-World Data (RWD) and Real-World Evidence (RWE) in the modern drug development landscape.
 View More
View More
13.11.2025
Public hospitals in Hong Kong now experience smoother initiation processes for industry-sponsored clinical studies, thanks to new Clinical Site Agreement (CSA) management initiative.
We are pleased to share the latest progress on the Clinical Site Agreement (CSA) management initiative, launched in April 2025 with full support from The University of Hong Kong Clinical Trials Centre (HKU-CTC).
 View More
View More